MolecularNeurobiologyResearch


Research interests
Chronic metabolic and neurodegenerative syndromes such as obesity or Parkinson’s disease, represent a growing epidemic especially in the constantly aging populations.
They result from a failure in the interplay of environmental factors, humoral signals, extra- and intracellular enzymatic cascades, genetic and epigenetic signals in the central nervous system and in the periphery.
Physiological and pathophysiological mechanisms involved in this regulation require intensive research to develop novel therapeutic strategies.
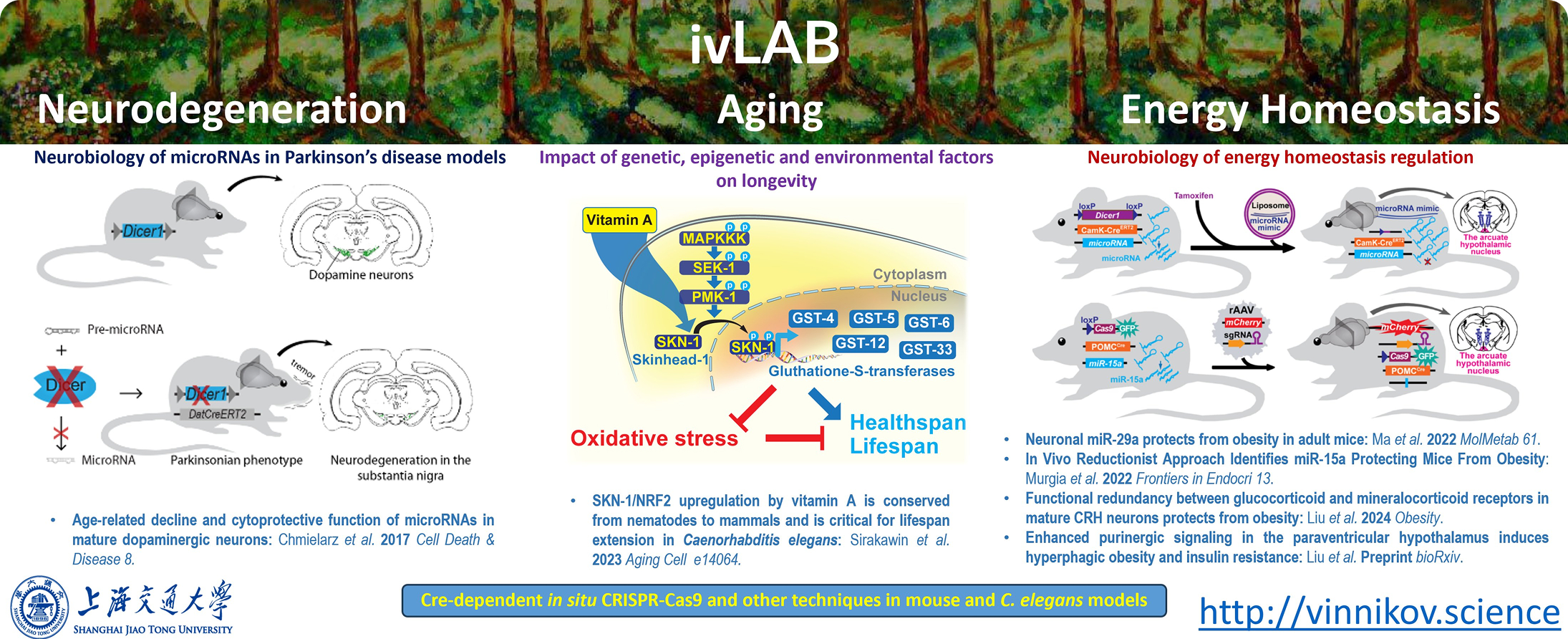
ivLAB has a strong interest in studying the roles of distinct cellular populations in physiology, aging and complex trait pathology
Modeling aging and age-related diseases in vivo
We use C. elegans and mice as in vivo models for circadian cycle, microbiome, nutrition, appetite regulation, aging and neurodegeneration research. For the first time, we have demonstrated an age-related decline of microRNAs and their critical physiological role in dopamine neurons (Chmielarz et al. 2017).
Exploring longevity pathways
Our studies in mice are complemented by mechanistic experiments in C. elegans. We have shown that micronutrients, such as vitamin A, can up-regulate SKN-1/NRF-2 transcription factor in ematodes and mice. SKN-1 pathway is critical for coping with oxydative stress and is critical for vitamin A-mediated longevity extension in C. elegans (Sirakawin et al, 2023).
Coding and non-coding gene manipulation by CRISPR-Cas9
Using an originally developed stabilized microRNA mimics delivery reductionist approach (Vinnikov et al. 2016, Najam et al, 2019, Murgia et al. 2022), we identified specific small RNAs expressed in the arcuate hypothalamic nucleus that protecting mice from obesity. The developmentally uncoupled self-inhibiting in situ CRISPR-Cas9 technique allowed us to validate the energy homeostasis regulating miR-29a-Nras circuit within the proopiomelanocortin neurons (Ma et al. 2022).
Regulation of energy homeostasis by the hypothalamus
For such Cas9-dependent approaches, we specifically designed an all-in-one tool to test in vitro the CRISPR on-target activity (Lin et al. 2024). Furthermore, we study the metabotropic circuits within the paraventricular nucleus of the hypothalamus. For example, we have recently discovered the critical metabotropic role of the functional redundancy of corticoid receptors in neurons expressing corticotropin-releasing hormone (Liu et al. 2024).
Novel pathological pathway within the hypothalamus induces obesity and insulin resistance
In our most recent study, we found that metabolic stress activated pathological purinergic circuit within the hypothalamus, which promotes hyperphagic obesity and insulin resistance. Moreover, nasal administration of clinically approved doses of inhibitors of this pathologic pathway counteracts diet-induced obesity and insulin resistance in mice and spontaneous weight gain in monkeys, paving the way for application of these compounds in patients with metabolic disorders (Liu et al, bioRxiv).
Selected Publications
Selective abundance of the stemness-promoting cluster miR-290-295 within the adult substantia nigra dopamine neurons is neuroprotective via preservation of protein synthesisLi Z, Xu Y, Murgia N, Kovzel N, Ng DS, Liu Y, Kang X, Jiang H, Domanskyi A, Vinnikov IA bioRxiv, preprint.
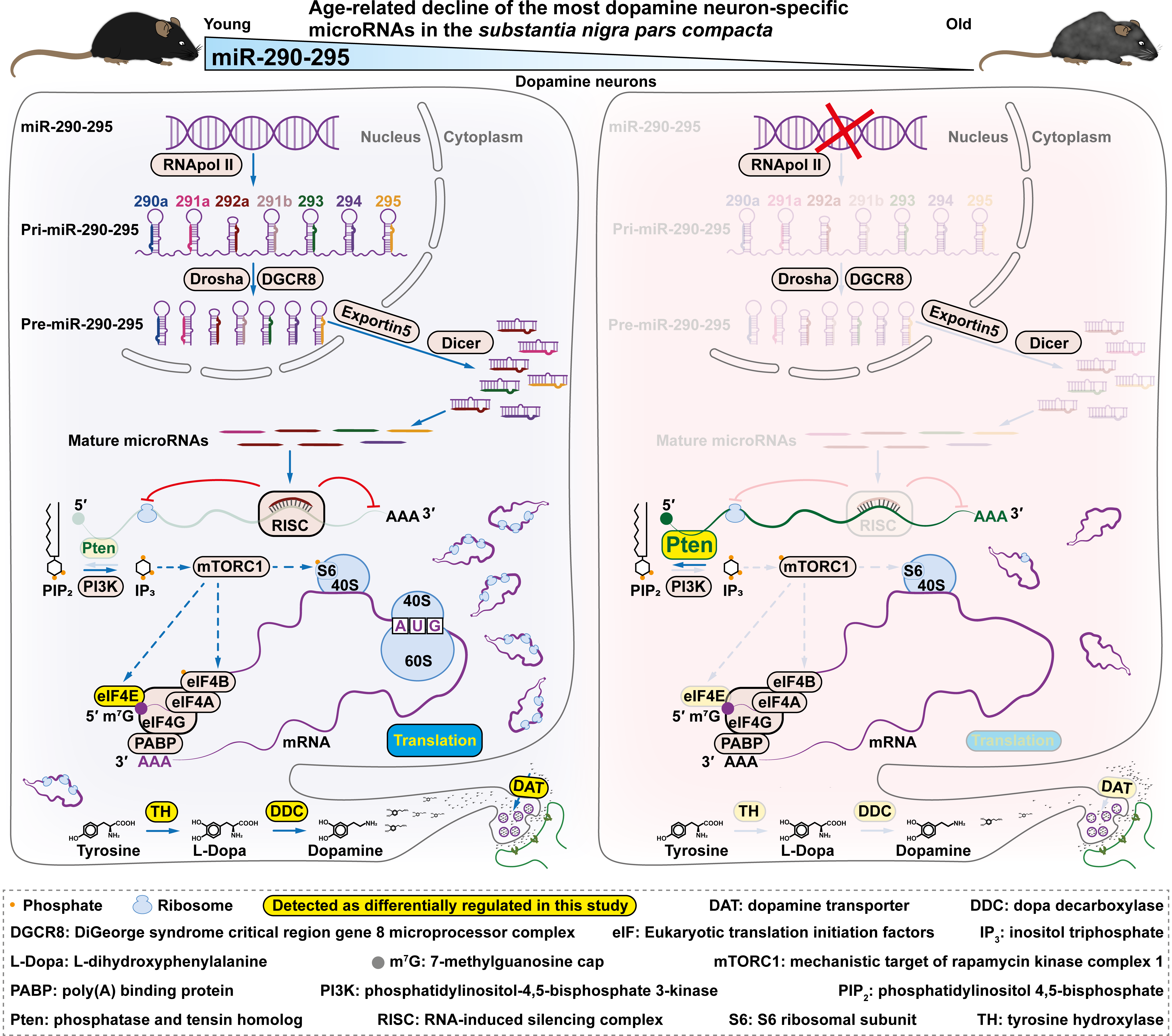
Locomotor, reward and other critical functions of the body are regulated by the ventral midbrain, with the central role played by dopamine (DA) neurons. The function of these cells from the early development to maturity is critically dependent on the orchestrated expression of coding and non-coding genes. For example, in the stem cells the miR-290-295 cluster constitutes the majority of expressed microRNAs and is critical for stemness in rodents. During development towards various terminally differentiated lineages, such as neurons, the cells typically switch off transcription of these stem cell-specific microRNAs. Here we report that within the adult substantia nigra pars compacta (SN), the miR-290-295 cluster is exclusively expressed in DA neurons (SNDA), preventing the locomotor deficits and maintaining an adequate expression of enzymes involved in DA biogenesis, such as tyrosine hydroxylase (TH), dopa decarboxylase (DDC) and DA transporter (DAT). Importantly, a global knock-out of the miR-290-295 cluster leads to decreased numbers of SNDA neurons in adult mice. Using in vitro and in vivo DA cell-specific loss-of-function models, we demonstrated that miR-292a-3p, the most abundant microRNA in this cluster, directly targets Pten, a phosphatase antagonizing the neuroprotective phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-Akt-mechanistic target of rapamycin kinase (mTOR) pathways regulating translation initiation. Mechanistically, when labelled with the click chemistry-compatible methionine analogue L-azidohomoalanine, miR-290-295 cluster-deficient SNDA neurons revealed a drastic impairment of protein synthesis, which is critical for DA biogenesis. Our surprising finding demonstrates for the first time a selective expression of stem cell-specific and stemness-promoting microRNAs in a distinct population of mature neurons to maintain their physiological functions in the adulthood, suggesting that similar epigenetic disinhibition mechanisms may be also critical for other terminally differentiated cells across species.
Remodeling of purinergic signaling in the paraventricular hypothalamus promotes hyperphagic obesity and insulin resistanceLiu Y, Bondarenko A, Zhang H, Luan P, Zhang Q, Wang B, Najam SS, Liang X, Syed AAS, Lin D, Huang S, Konovalov R, Konopka W, Zhang Z, Zhang Y, Wu S, Jing M, and Vinnikov IA bioRxiv, preprint.
Astrocytes in the hypothalamus sense metabolic cues and emit ATP to signal to neighboring cells.
This story opens a new field of context-dependent pathological purinergic signaling within the hypothalamus, paving an avenue for novel therapeutic approaches to treat metabolic disorders.
Recently, we found that high fat diet (HFD) feeding dose-dependently increases the frequency of bursts of ATP release called Inflares in the mouse paraventricular hypothalamic nucleus (PVH). This phenomenon was also confirmed for PVH of hyperglycemic mice.
We then hypothesized that, in addition to P2Y12 receptor-expressing microglia, this Inflare signaling can be also decoded by other cells in this region. Indeed, we demonstrated that HFD exposure or diabetes mellitus lifts the epigenetic restriction to „ectopically“ express this hematopoietic lineage-specific marker in a subset of neurons expressing oxytocin. Strikingly, this „ectopic“ up-regulation can be observed also in oxytocin neurons of diabetic human patients.
Further, we validated both in vitro and in vivo that P2Y12 counteracts c-Fos expression and promotes hyperphagic obesity and insulin resistance. As such, we hypothesized that inhibition of this ATP/ADP receptor in HFD-fed mice may reverse these metabolic phenotypes.
To do that, we established a nasal route of delivery of P2Y12 inhibitors in doses clinically approved for humans which indeed protected both mice and macaques from obesity and insulin resistance.
Functional redundancy between glucocorticoid and mineralocorticoid receptors in mature CRH neurons protects from obesityLiu Y, Bondarenko A, Zhang H, Luan P, Zhang Q, Wang B, Najam SS, Liang X, Syed AAS, Lin D, Huang S, Konovalov R, Konopka W, Zhang Z, Zhang Y, Wu S, Jing M, and Vinnikov IA bioRxiv, preprint.

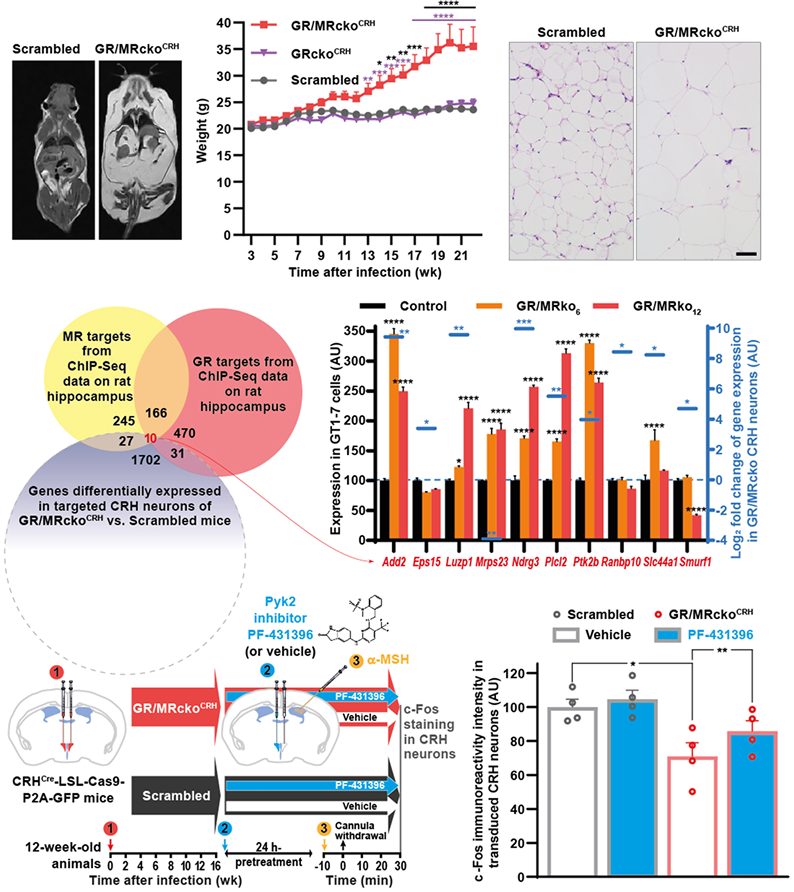
This story demonstrates that mineralocorticoid receptors (MR) within the neurons expressing corticotropin-releasing hormone (CRH) can compensate for the loss of glucocorticoid receptor (GR) to protect mice from obesity.
Similar to double-knockout models targeting forebrain neurons or the paraventricular hypothalamic nucleus, simultaneous knock-out of both GR and MR in CRH neurons leads to obesity.
This redundancy of corticoid receptors is critical to counteract Pyk2 expression and thus maintain the responsiveness of these neurons to melanocortinergic signaling.
SKN-1/NRF2 upregulation by vitamin A is conserved from nematodes to mammals and is critical for lifespan extension in Caenorhabditis elegansSirakawin C, Lin D, Zhou Z, Wang X, Kelleher R, Huang S, Long W, Pires-daSilva A, Liu Y, Wang J, and Vinnikov IA 2023. Aging Cell, 00:e14064.

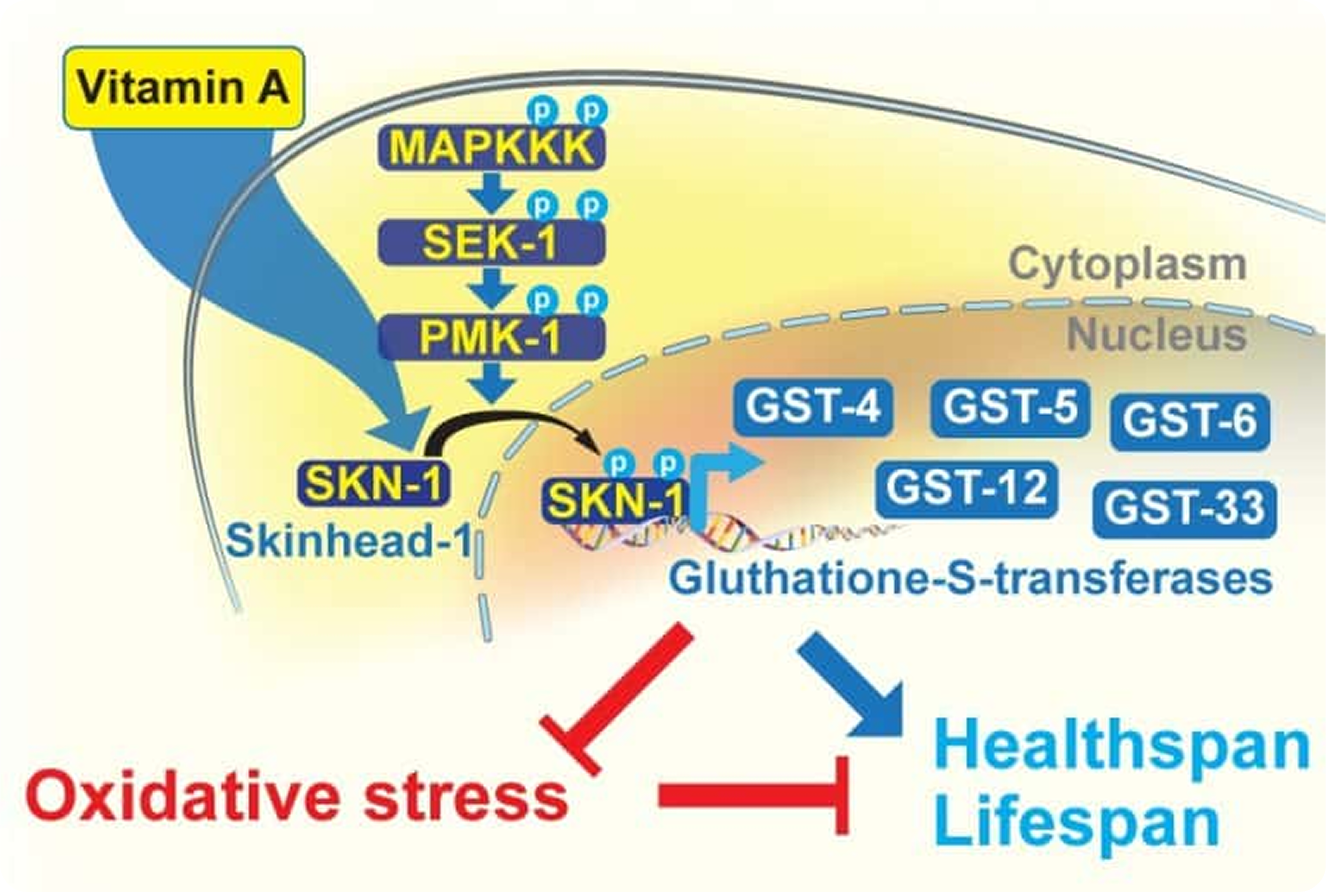
Vitamin A (VA) is a micronutrient essential for the physiology of many organisms, but its role in longevity and age-related diseases remains unclear.
In this work, we used Caenorhabditis elegans to study the impact of various bioactive compounds on lifespan. We demonstrate that VA extends lifespan and reduces lipofuscin and fat accumulation while increasing resistance to heat and oxidative stress.
This resistance can be attributed to high levels of detoxifying enzymes called glutathione S-transferases, induced by the transcription factor skinhead-1 (SKN-1). Notably, VA upregulated the transcript levels of skn-1 or its mammalian ortholog NRF2 in both C. elegans, human cells, and liver tissues of mice.
Moreover, the loss-of-function genetic models demonstrated a critical involvement of the SKN-1 pathway in longevity extension by VA.
Our study thus provides novel insights into the molecular mechanism of anti-aging and anti-oxidative effects of VA, suggesting that this micronutrient could be used for the prevention and/or treatment of age-related disorders.
Noodles, the all-in-one system for on-target efficiency analysis of CRISPR guide RNAsLin D, Najam S S, Liu Y, Murgia N, and Vinnikov IA 2024. MethodsX, 12, 102481.
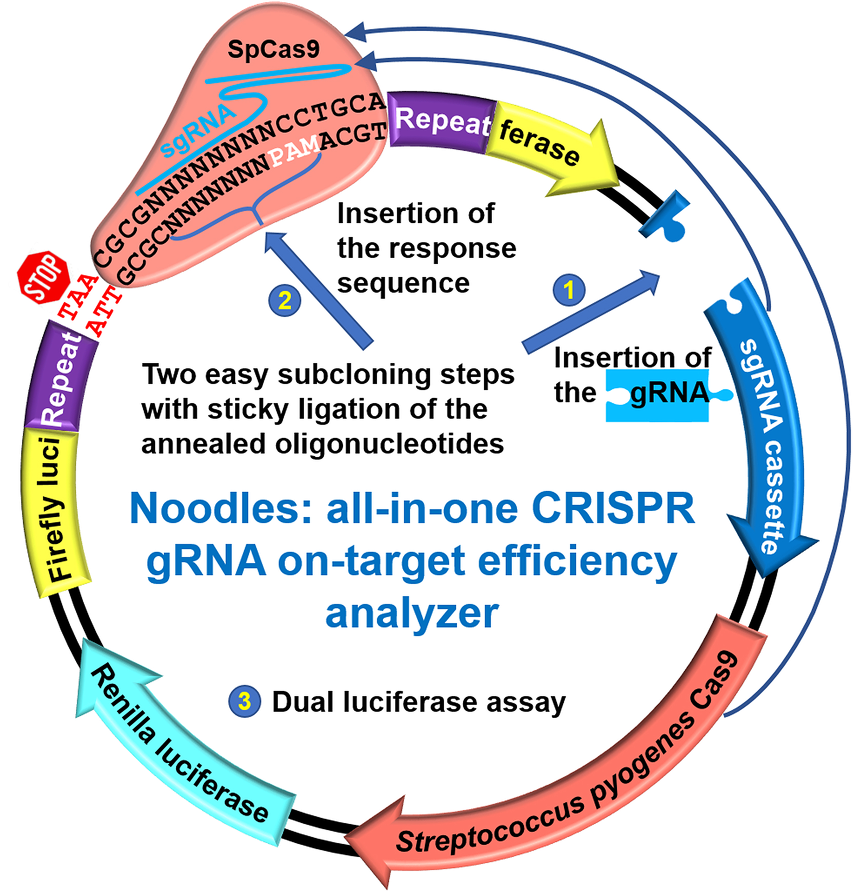
The efficiency of clustered regularly interspaced short palindromic repeats (CRISPR) guide RNA (gRNA) targeting is critical for CRISPR associated protein 9 (Cas9)-dependent genomic modifications.
Here, we developed Noodles, an all-in-one system to test the on-target activity of gRNAs easily and efficiently. Single-strand annealing repair mechanism of the split luciferase gene allows a positive selection of gRNAs efficiently driving nuclease activity of Cas9 from Streptococcus pyogenes (SpCas9).
Our system can reliably validate in silico -predicted gRNAs before implementing them for in vitro and in vivo applications.
Altogether, Noodles might be an asset for researchers and bioengineers, saving their time and efforts, while keeping the screening efficient and sensitive.
- All-in-one dual-luciferase system to easily probe on-target activity of gRNAs based on homology-directed repair mechanism.
- Easy-to-subclone spCas9-based 2-plasmid system comprising Renilla luciferase for transfection efficiency control.
Ma Y, Murgia N, Liu Y, Li Z, Sirakawin C, Konovalov R, Kovzel N, Xu Y, Kang X, Tiwari A, Mwangi PM, Sun D, Erfle H, Konopka W, Lai Q, Najam SS and Vinnikov IA 2022. Molecular Metabolism, https://doi.org/10.1016/j.molmet.2022.101507.
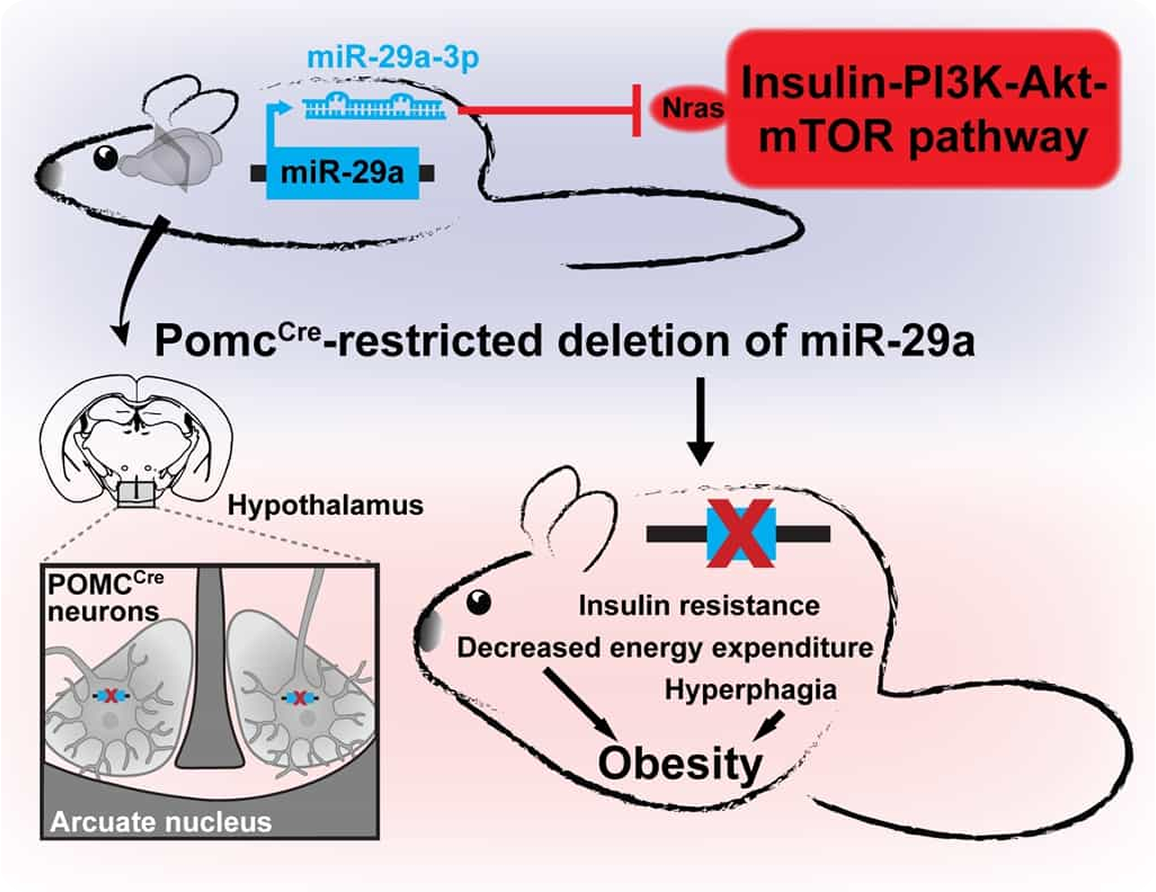
This is a pioneering study discovering that the loss of only one microRNA in a specific neuronal population can lead to severe metabolic phenotype in adult mice.
In a view of our previous data that loss of Dicer, a critical nuclease for microRNA maturation, in mature forebrain neurons induces obesity via hyper-activation of insulin-PI3K-Akt-mTor pathway, we used several innovative approaches to achieve spatial and target specificities.
To localize the region of interest, we employed stereotaxic injections of Cre recombinase-equipped rAAVs to different regions of Dicer1fl/fl animals followed by unprecedented developmentally uncoupled Cre-dependent self-inhibiting in situ CRISPR-Cas9 technique. The latter approach employed CRISPR gRNAs to cleave not only the gene of itnerest, but also Cas9 in neurons of proopiomelanocortin lineage to decrease off-targeting.
Regarding the target specificity, unbiased in vitro antagomiR screening to identify microRNAs targeting insulin-PI3K-Akt-mTor pathway was complemented by in vivo pharmacological reductionist approach. For the latter, we predicted microRNAs targeting the above-mentioned pathway and delivered the mixture of stabilized microRNA mimics to the arcuate nucleus of Dicer-depleted mice to attenuate obesity.
Further, we iteratively divided the group of oligonucleotides into subgroups and identified the specific miR-29a that was capable to attenuate obesity in mice. Strikingly, obesity induced by deletion of miR-29 in proopiomelanocortin lineage neurons could be attenuated by a simultaneous knockout of its direct target Nras, validating its contribution to the observed phenotype.
- Delivery of miR-29a-3p to the arcuate hypothalamic nucleus attenuates obesity.
- Knock-out of genes in mature neurons by Cre-dependent CRISPR/Cas9 technique involving Cas9-cleaving sgRNAs to limit off-target effects.
- Deletion of miR-29a in mature POMCCre neurons leads to early-onset insulin resistance.
- POMCCre-restricted deletion of miR-29a causes cell-autonomous Nras up-regulation leading to obesity associated with both energy intake and expenditure.
- POMCCre-restricted knock-out of Nras, a direct target of miR-29a-3p, attenuates obesity in mice.
Lai Q, Kovzel N, Konovalov R and Vinnikov IA 2021. Adv Exp Med Biol 1208: 191-264.2016.
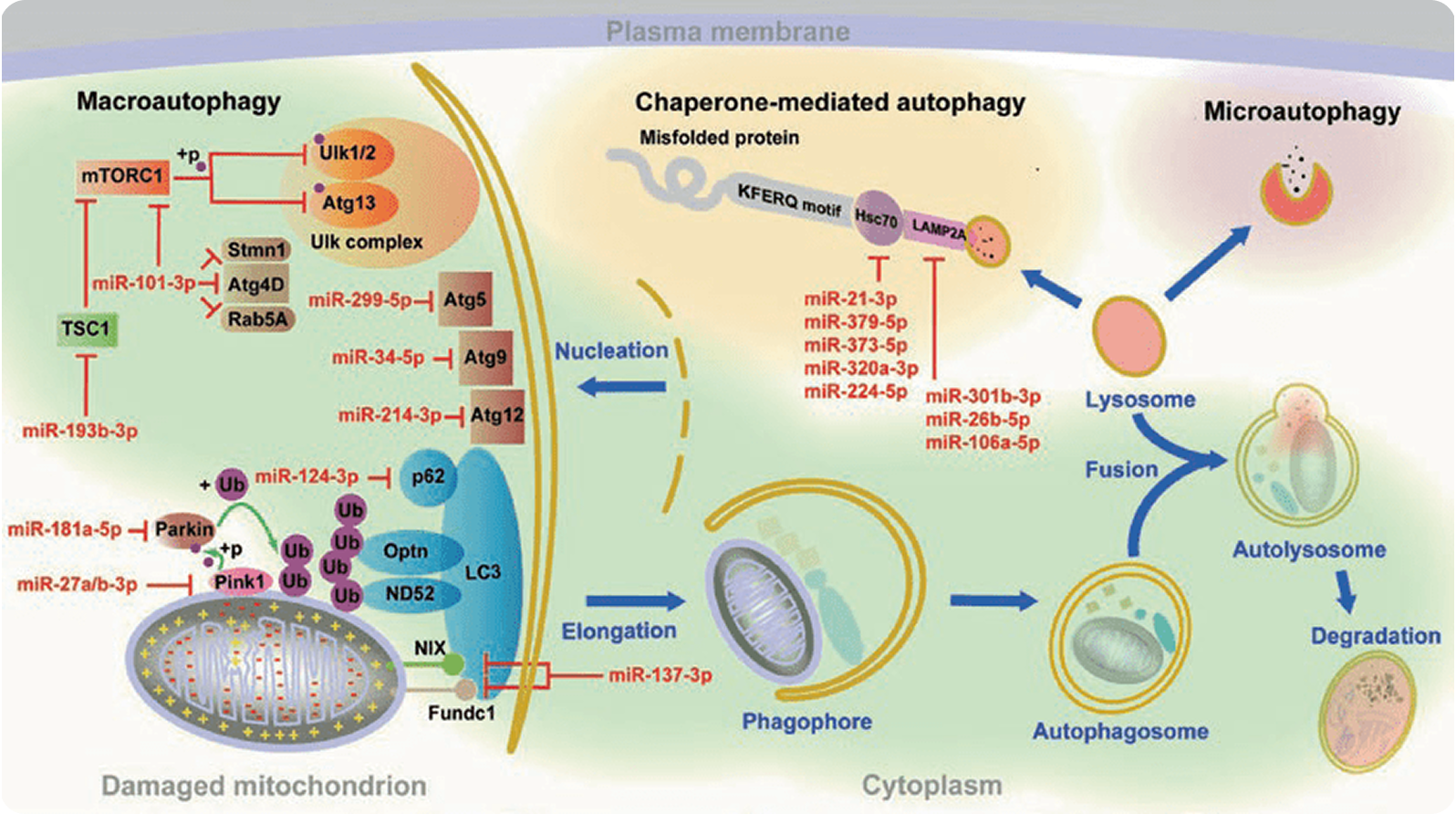
Social and economic impacts of neurodegenerative diseases (NDs) become more prominent in our constantly aging population. Currently, due to the lack of knowledge about the aetiology of most NDs, only symptomatic treatment is available for patients. Hence, researchers and clinicians are in need of solid studies on pathological mechanisms of NDs.
Autophagy promotes degradation of pathogenic proteins in NDs, while microRNAs post-transcriptionally regulate multiple signalling networks including autophagy. This chapter will critically discuss current research advancements in the area of microRNAs regulating autophagy in NDs.
Moreover, we will introduce basic strategies and techniques used in microRNA research. Delineation of the mechanisms contributing to NDs will result in development of better approaches for their early diagnosis and effective treatment.
Roles of microRNAs in Parkinson’s and other neurodegenerative diseasesLai Q, Murgia N, Parkkinen I, Domanskyi A and Vinnikov IA 2019. In B. Mallick (Ed.), AGO-Driven Non-Coding RNAs: Academic Press 8 (pp. 209-232).
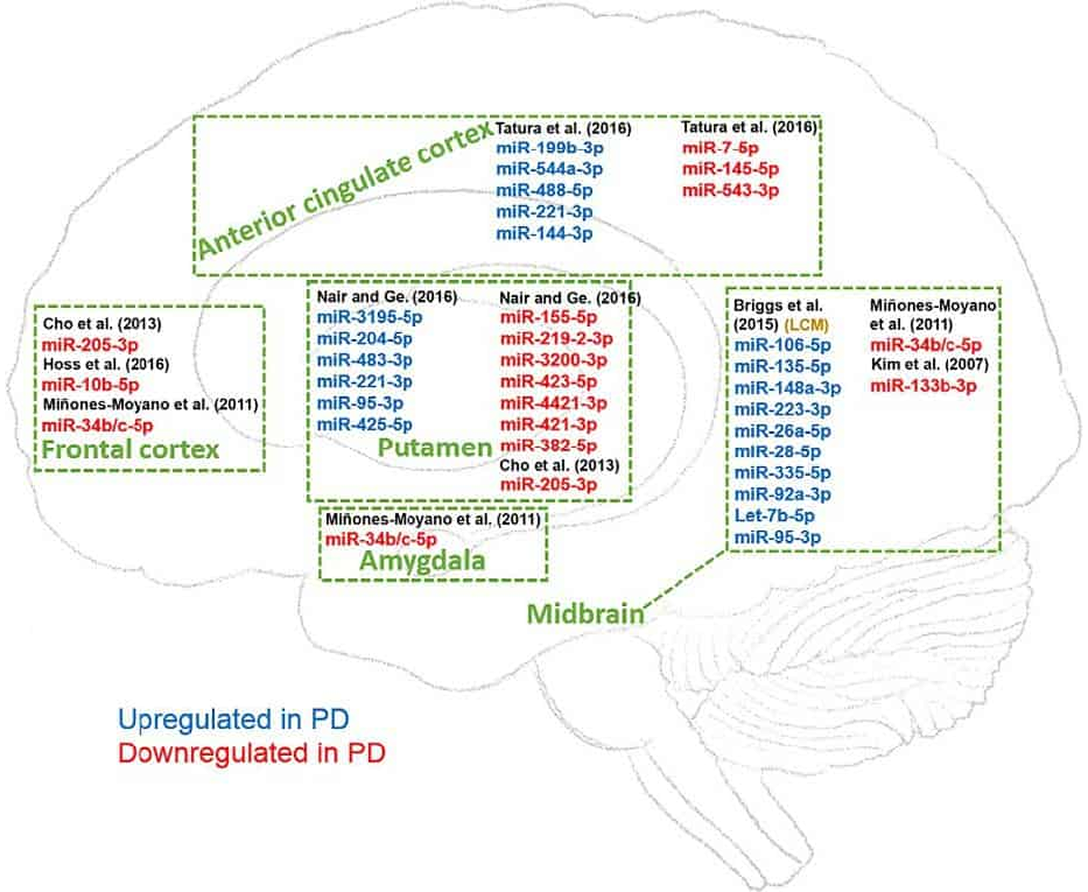
Multiple factors contribute to the onset and progression of neurodegenerative (ND) diseases including genetic mutations, posttranscriptional dysregulation, and environmental influence.
MicroRNAs are small noncoding RNAs adjusting expression of genes participating in the development and physiology of many tissues including central and peripheral nervous systems. Due to the unique characteristics of neurons including local translation and RNA interference (RNAi) in the neurites, microRNAs play a crucial role in neuronal physiology and pathophysiology.
Here, we highlight the age-related and disease-specific expression changes of neuronal microRNAs and their possible roles in Parkinson’s disease (PD) and other ND diseases.
MicroRNAs in the hypothalamic control of energy homeostasisNajam SS, Zglinicki B, Konopka W and Vinnikov IA 2019. Cell and Tissue Research 8: 375(1), 173-177.
Obesity is a medical condition characterized by excess of fat content in the body. The majority of obese patients additionally develop cardiovascular disease and type 2 diabetes mellitus collectively referred to as the metabolic syndrome. Such condition reduces life expectancy and may ultimately result in cancer development. It affects more than half a billion adults worldwide and is a constantly growing epidemic, especially in developed and developing countries.
This can be largely attributed to the lack o fgenetic, epigenetic, and social resistance to the unprecedented availability of cheap carbohydrate and fat rich food. Metabolic challenges such as overeating or fasting transiently or chronically disrupt energy homeostasis which is maintained by a tightly regulated communication between peripheral organs and the central nervous system.
In the former, the key regulatory functions are attributed to the pancreas, the main endocrine organ in the glucose metabolism, fat-storing cells called adipocytes, and the liver, the metabolic factory of our body.
Integration of all signals from the peripheral organs and control of feeding behavior and weight are governed by the brain, where the major regions involved in metabolism are located within the hypothalamus. It contains a number of nuclei crucial for hunger and satiety responses.
The homeostatic balance is achieved at several levels: (i) hormonal, by soluble factors signaling throughout the body; (ii) neuronal, by a complex network of neural cells; (iii) transcriptional, regulation of gene message expression in the cell nucleus; and (iv) at the post-transcriptional level, where mRNA and protein levels are regulated outside of the cell nucleus.
MicroRNAs represent a well-characterized class of small non-coding RNAs which can degrade gene messages or abrogate protein translation. In this work, we briefly reviewed the role of microRNAs in the energy homeostasis control by the hypothalamus.
Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression?Vinnikov IA, Domanskyi A 2017. Neural Regeneration Research 12, 1602-1604.
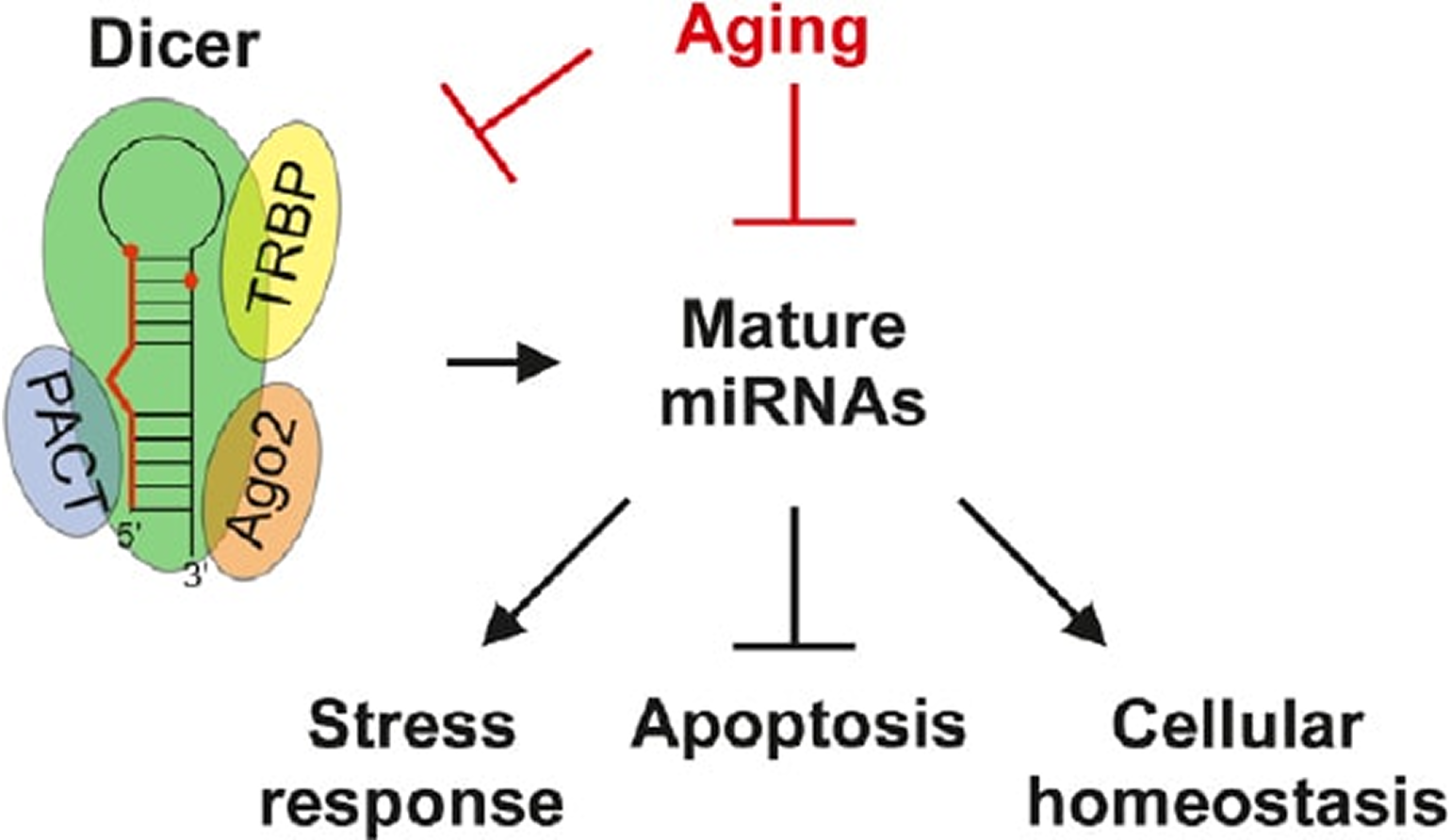
MicroRNA pathway is down-regulated in aged dopaminergic neurons: Parkinson’s disease (PD) is the most frequent motor neurodegenerative disorder and is morphologically mainly associated with progressive dopaminergic neuronal loss in the ventral midbrain. The cause of this pathology is unknown, but aging is well established as the strongest risk factor, which by far prevails over gender, environmental and genetic factors.
In our recent work (Chmielarz et al., 2017), we have demonstrated that the expression of Dicer, a multidomain ribonuclease III and a key endonuclease in microRNA (miRNA) maturation pathway, is significantly down-regulated in aged mouse midbrain.
Further, using a laser-assisted microdissection and quantitative PCR profiling techniques, we analyzed microRNAomes of dopaminergic neurons from the mouse substantia nigra and identified a predominant decrease of miRNA expression in aged dopaminergic neurons.
Dicer mRNA levels are also reduced in the ventral midbrain of PD patients (Simunovic et al., 2010). Importantly, several miRNAs have been shown to regulate PD-associated genes, suggesting that age-related deregulation of miRNA signaling may contribute to neurodegeneration (Heman-Ackah et al., 2013).
The two major questions arising from these observations are:
- Does the miRNA-mediated regulation provide an essential protection mechanism from neurodegeneration?
- May this protective component be compromised during aging and make the dopamine system more susceptible for other genetic and environmental factors leading to PD?
In our studies, we attempt to resolve these two challenges. Recently, we have found a way to answer the first of these two major questions: miRNA pathway indeed turned out to be cytoprotective for adult dopaminergic neurons (Chmielarz et al., 2017).
An increasing number of published and ongoing studies also start to address the second question, identifying neuronal functions- and survival-regulating genes and pathways targeted by miRNA network in the context of neurodegeneration (Briggs et al., 2015).
Dicer and microRNAs protect adult dopamine neuronsChmielarz P, Konovalova J, Najam SS, Alter H, Piepponen TP, Erfle H, Sonntag KC, Schütz G, Vinnikov IA, Domanskyi A 2017. Cell Death and Disease 8(5): e2813.
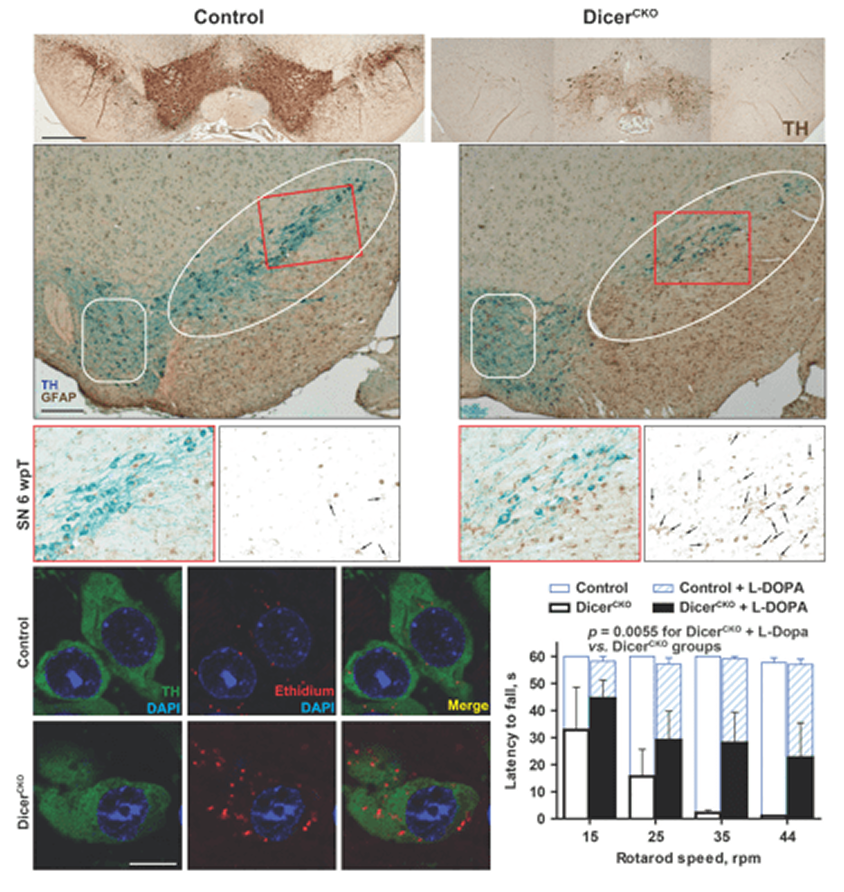
In this study, using laser-assisted dissection of dopamine neurons, we discovered an age-related decline of microRNAs in these cells.
Moreover, in the same work we demonstrated that tamoxifen-dependent knock-out of Dicer is lethal for these neurons, leading to rapid devastation of the ventral mitbrain accompanied by morphological, molecular and behavioral hallmarks of parkinsonism-like phenotype.
Vinnikov IA, Domanskyi A, Domanskyi A and Konopka W 2016. physiology (Neuromethods), Humana Press. 7: 9.
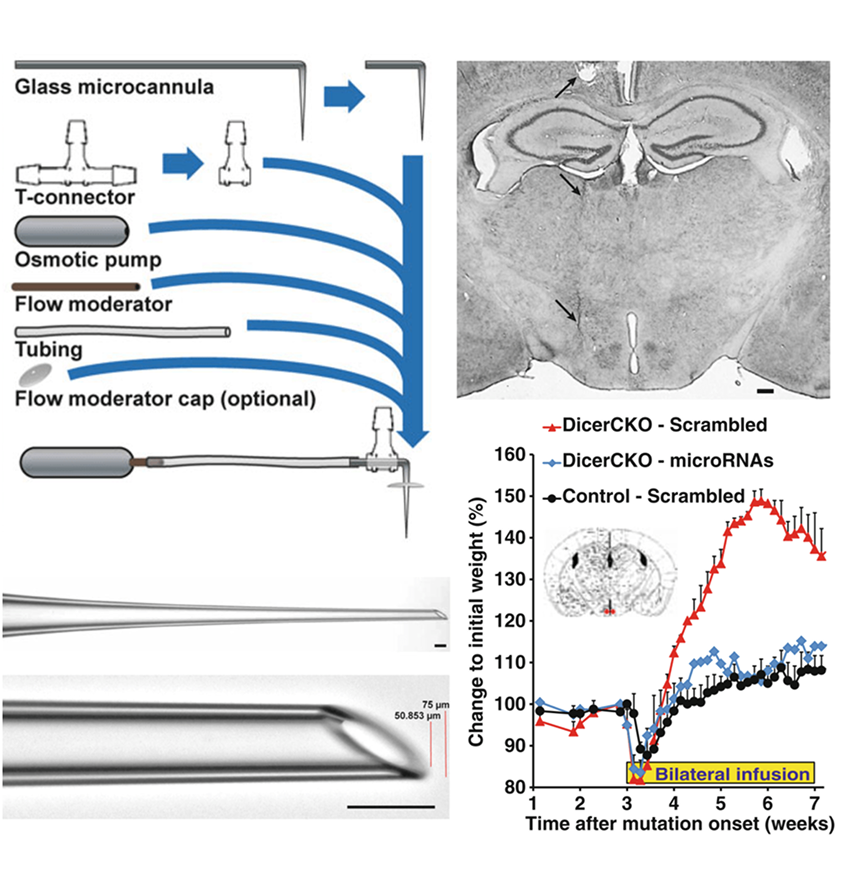
The growing field of RNA neurobiology dictates development and improvement of effective and reliable in vivo techniques to address the function of particular microRNA molecules within the brain.
Here we describe a novel method involving continuous delivery of oligonucleotides into a brain region of interest by osmotic pump infusion. The approach implements application of double-stranded microRNA-mimics with only two LNA moieties at the 30-end and additionally one at the 50-end of the sense strand.
This method holds promise for long-lasting and specific siRNA upregulation in vivo, especially in the Dicer-depleted systems, where other approaches are limited or not applicable.
Being robust and effective, various techniques described in this chapter can be easily modified in order to achieve up- or downregulation of expression of specific RNA molecules, bi- or unilateral infusions or injections, and in vivo “screening” strategy allowing to start from a bigger group of RNA molecules and end up with identification of single RNA species critical for a phenotype.
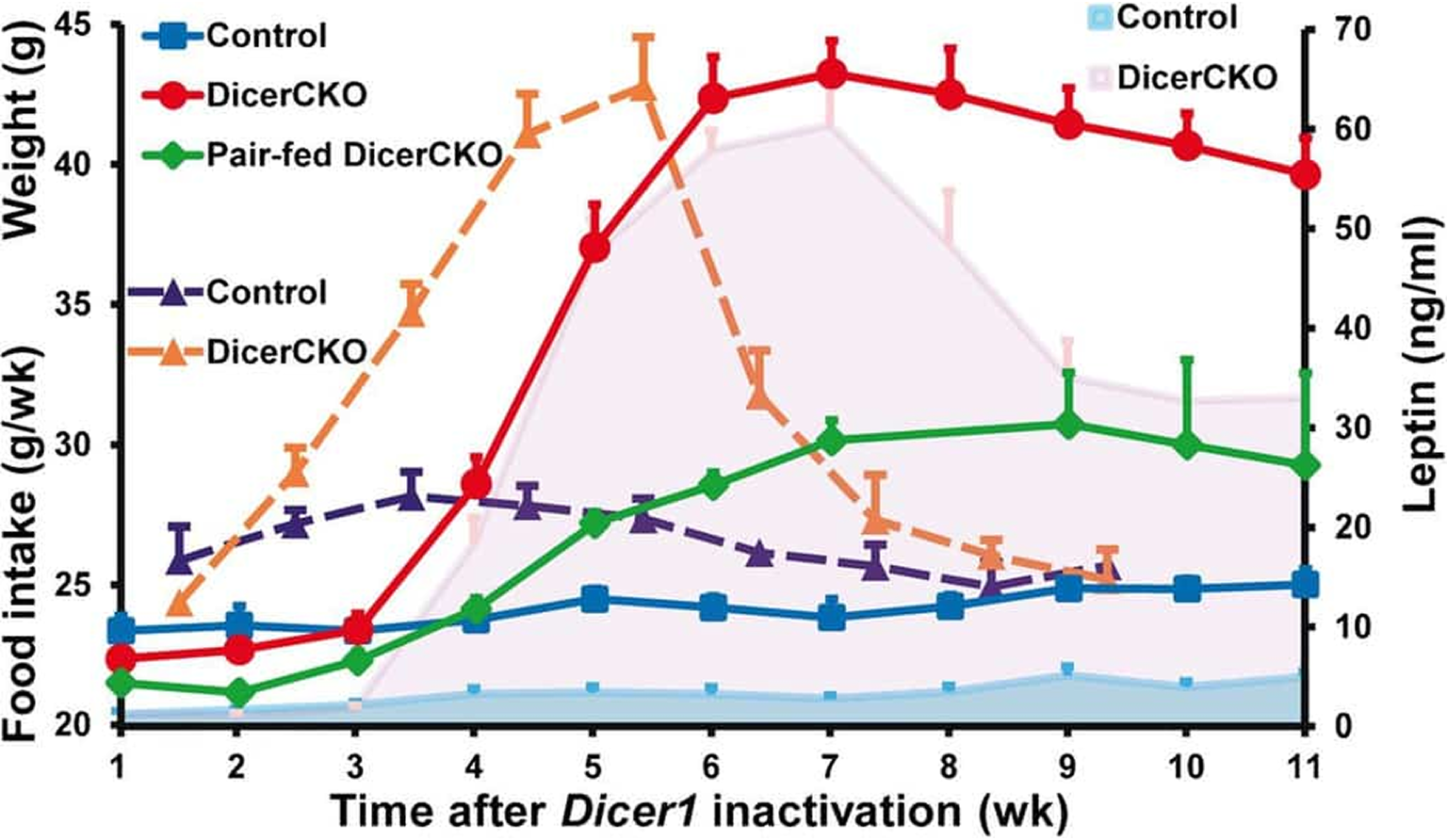
Hypothalamic miR-103 Protects from Hyperphagic Obesity in MiceVinnikov IA, Hajdukiewicz K, Reymann J, Beneke J, Czajkowski R, Roth LC, Novak M, Roller A, Dörner N, Starkuviene V, Theis FJ, Erfle H, Schütz G, Grinevich V and Konopka W 2014. The Journal of Neuroscience 34(32): 10659-10674.
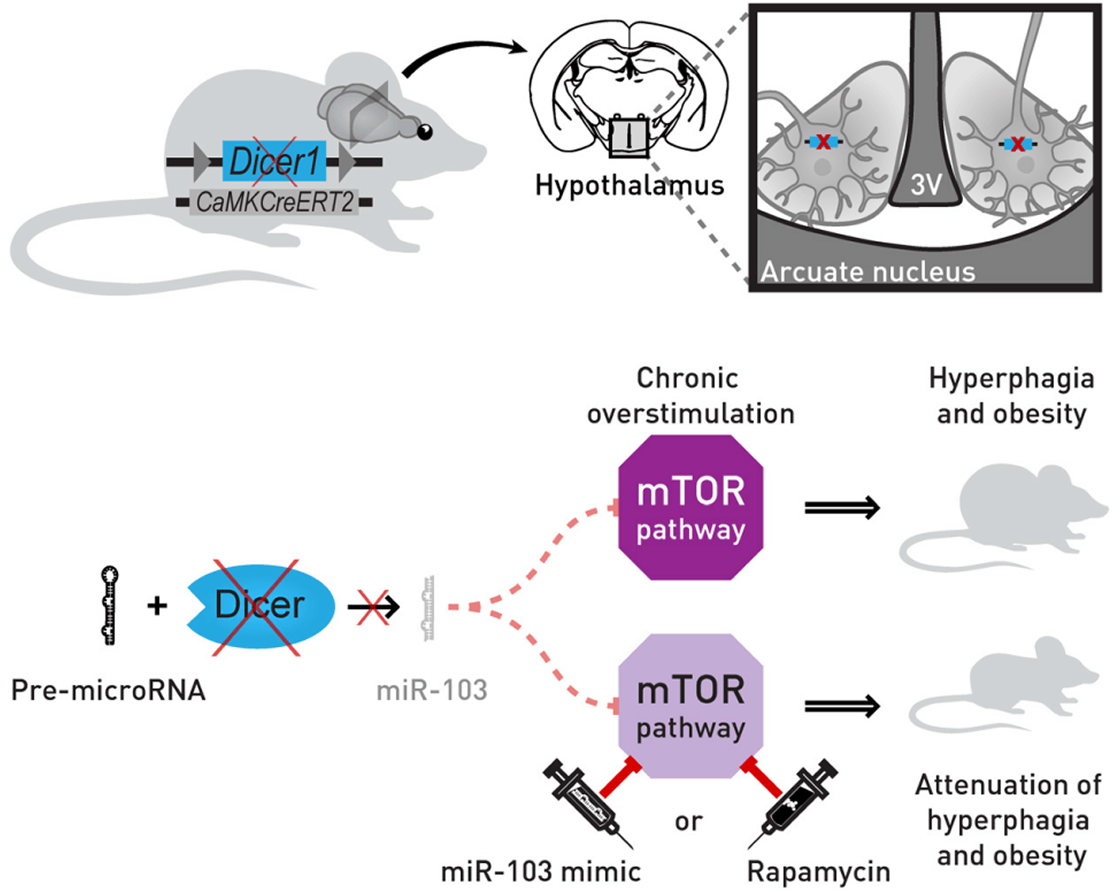
The role of neuronal non-coding RNAs in energy control of the body is not fully understood. The arcuate nucleus (ARC) of the hypothalamus comprises neurons regulating food intake and body weight.
Here we show that Dicer-dependent loss of microRNAs in these neurons of adult (DicerCKO) mice causes chronic overactivation of the signaling pathways involving phosphatidylinositol-3-kinase (PI3K), Akt, and mammalian target of rapamycin (mTOR) and an imbalance in the levels of neuropeptides, resulting in severe hyperphagic obesity.
Similarly, the activation of PI3K-Akt-mTOR pathway due to Pten deletion in the adult forebrain leads to comparable weight increase. Conversely, the mTORC1 inhibitor rapamycin normalizes obesity in mice with an inactivated Dicer1 or Pten gene.
Importantly, the continuous delivery of oligonucleotides mimicking microRNAs, which are predicted to target PI3K-Akt-mTOR pathway components, to the hypothalamus attenuates adiposity in DicerCKO mice.
Furthermore, loss of miR-103 causes strong upregulation of the PI3K-Akt-mTOR pathway in vitro and its application into the ARC of the Dicer-deficient mice both reverses upregulation of Pik3cg, the mRNA encoding the catalytic subunit p110γ of the PI3K complex, and attenuates the hyperphagic obesity.
Our data demonstrate in vivo the crucial role of neuronal microRNAs in the control of energy homeostasis.
Click here to see the full publication list
Contacts
Ilya A. Vinnikov
Associate Professor
Head, Laboratory of Molecular Neurobiology
Sheng Yushou Center of Cell Biology and Immunology
Department of Genetics and Developmental BiologySchool of Life Sciences and BiotechnologyShanghai Jiao Tong University 800 Dongchuan Road,
Shanghai, 200240, China

Download PDF-version of CV and publications

